
Ethylene oxide (EO) is an organic compound with the chemical formula C2H4O. It is a known carcinogen and is often used as a sterilizer. Relevant regulations require regular testing in production and use. Ethylene oxide residue According to GB/T 16886.7-2015 biological Evaluation of Medical Devices part 7: Ethylene oxide residue sterilization GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment test method Part 1: Chemical Analysis Methods, GB19083-2010 Technical Requirements for Medical Protective Masks, ISO10993-7; 2008 “ethylene oxide (EO) and 2-chloroethanol (ECH) residue detection” in the gas chromatography for detection.
Our company’s ethylene oxide (EO) detection solution is to quantitatively determine the content of EO in the sterilized medical equipment with headspace air chromatograph. The instrument configuration of this solution is reasonable. Simple operation is a practical testing scheme for medical equipment manufacturers.
1.Detection index
ethylene oxide (EO)
2.Reagent
Ethylene oxide (EO) standard
3.Experimental conditions
Gas phase detection conditions: column temperature 60℃, vaporization chamber 220℃, FID detector 250℃
Headspace sampling conditions: sample heating temperature 60℃, valve box temperature 100℃, pipeline temperature 120℃
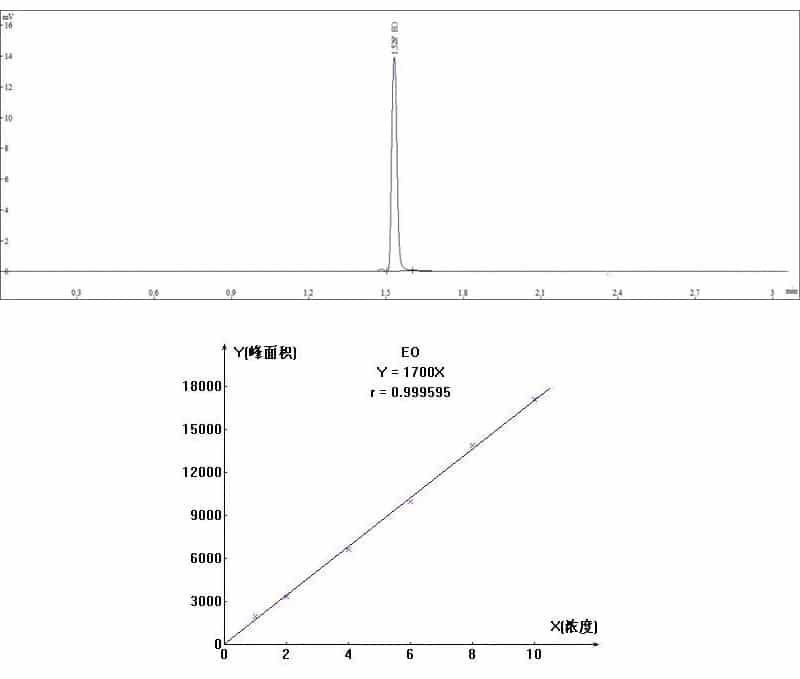
4.Spectra data
Headspace injection volume was 1mL, and internal standard method was used for analysis.
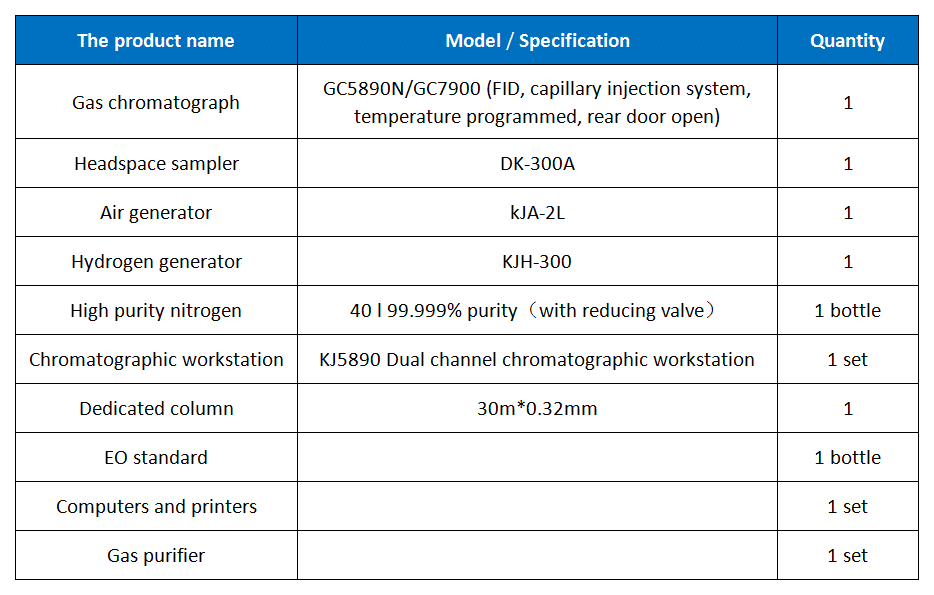
5.Instrument Configuration

We are a branch of Kejie holding group, in charge of the overseas marketing of Kejie products, providing to customers with chromatograph, mass spectrometry, spectrum and other analytical scientifc instruments and professional services all around the world.Welcome to visit our website: www.changior.com or contact us via email: sales@changior.com
